Abstract
Enclosure in a cell wall and the resulting immobility of plant cells compels plants to particular modes of development. Plants develop entirely by cell growth and division, lacking any means of cell migration while cell death occurs only in development of vascular tissues. In spite of being much more constrained in their development than complex multicellular organisms with mobile cells lacking walls, plants develop according to basic body plans and plastically modify their development to best accommodate what information they obtain about its environment. Development in plants beyond embryogenesis proceeds through meristems, necessitating mechanisms for maintenance of meristems and suppression of lateral buds. These principles of development of a basic body plan in plants will here be reviewed, using mostly evidence from the model system Arabidopsis, while refraining from exploring the details of development of individual plant organs, such as roots, leaves and flowers.
Introduction
Plants, except in the development of xylem, rely on cell division and shape change only to enforce a body plan. Accordingly, they exercise great control about each of these processes. This essay discusses sequentially the plant cell wall, embryonic patterning, the development and maintenance of meristems and the maintenance of apical dominance, thus covering the biochemistry, molecular cell biology and molecular genetics of how plants establish their respective body plans.
Constraint and opportunity - the plant cell wall
Besides serving as the main support system of plants in a terrestrial environment, protecting cells and providing the apoplast pathway for transport of fluids through plants, the properties of plant cell walls are also essential to understanding plant growth.
Only the semirigid primary cell wall allows cell growth, and is often complemented with a secondary cell wall being laid down inside the primary one to conclude differentiation when growth is complete. Cellulose microfibrils, the principal constituent of cell walls, are laid down roughly parallel to each other in each layer (lamella), lamellae being deposited inside one another by rosette-shaped, multi-subunit molecules of cellulose synthase in the cell membrane (1). It is thought that microtubules restrict the movement of cellulose synthase complexes within the cell membrane to effect this parallel orientation (2-4). The major components of the matrix around microfibrils are hemicelluloses and pectins (5). Molecules of the hemicellulose group bind cellulose microfibrils and pectins. Extensin proteins add an additional unit of scaffolding to the matrix. Many of the bonds between constituents of the cell wall are noncovalent and hence easily manipulated when growth is required (5).
Plant cell growth is said to happen when the turgor pressure exceeds the resistance (yield threshold) of the cell wall6. Plant cells will typically elongate in a specific direction (anisotropic growth). Anisotropic growth is achieved if the yield threshold is non-uniform over the cell surface. There are two factors contributing to non-uniformity in cell elongation: Orientation of microfibrils and relaxation of the cross-links within the wall (6).
The microfibrils within a lamella form rings or spirals around the cell, allowing only growth in the direction perpendicular to the orientation of microfibrils (7). Under the influence of phytohormones such as ethene and gibberellic acid the microfibrils in the cell wall can reorganise in a different direction, allowing control over the direction of growth (Figure 1).
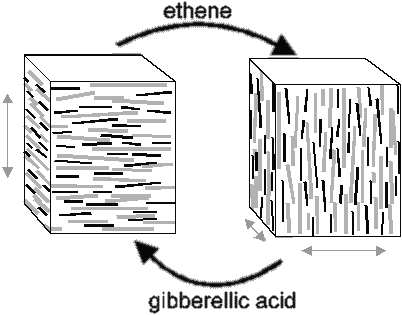 | Figure 1: Environmental cues effect changes in microtubule and microfibril orientation. |
Cross-links between cellulose microfibrils in particular have to be relaxed before elongation of primary cell walls, and hence cell growth, can occur. As a mechanism how some of these bonds can be relaxed, the acid growth hypothesis (8) has been proposed, stating that the phytohormone auxin stimulates localised proton secretion by indirect or direct interaction with a proton pump in the plasma membrane. The resulting local change in pH (from 7.0 to 4.5) activates expansin proteins in the wall (9). These disrupt the hydrogen bonding between cellulose microfibrils, allowing the latter to drift apart as turgor pressure exceeds yield threshold (10). The turgor pressure required for plant cells to elongate is generated by a hypertonic vacuole. As the plant cell elongates, the vacuole enlarges, whilst the volume of cytosol remains constant (11).
Of interest to the study of plant development is also the fact that the cytosols of adjacent plant cells are usually connected through plasmodesmata, allowing efficient diffusion of chemical signals (12-14), and the suggestion that plant cell walls may serve as a developmental memory, as they do in Fucus (15).
Box 1: Early development of ArabidopsisThe asymmetric division of the zygote gives rise to the embryo and the extra-embryonic suspensor, through which nutrients are transported to the embryo (46). The suspensor also pushes the embryo into the lumen of the embryo sac (47). In later development the uppermost cell of the suspensor gives rise to the hypophysis of the embryo (48), which takes part in developing the root cap and tip. The embryo is simple and lacks some structures present in the mature plant. However, the shoot apical meristem, from which all later above-ground meristems are derived, becomes established at this stage. The various appearance of the embryo at various stages has been used to provide a context for discussing developmental events. Their names are given here. | 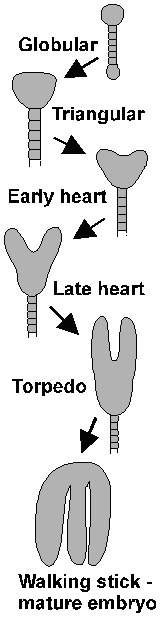 |
Embryology
As the plant embryo develops from the zygote, two developmental patterns have to be established: The apical-basal differentiation gives the embryo a root and a shoot end. The radial pattern determines three concentric cell layers each of predetermined fate (Figure 2). Pattern formation, the process that allows organs to arise in the correct positions on the apical-basal axis, is seen as distinct from morphogenesis, the cellular processes of oriented cell division and cell shape change (16). The main approach to understanding such processes is mutant analysis. Mutants deficient in either apical-basal or radial patterns are known, as well as segment deletion mutants.
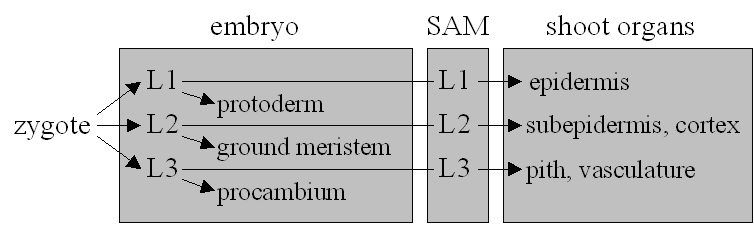 |
| Figure 2: The fundamental cell layers of Angiosperms and tissues derived from them in the embryo and shoot. |
One recent discovery was that of a maternal effect on pattern formation in the seed. Reports of embryos developing in ovules of short integument1 mutants range from funnel-shaped cotyledons to masses of undifferentiated cells (17). It remains unclear whether SHORT INTEGUMENT1 is required for proper apical-basal patterning of the embryo or whether the embryonic development is simply distorted because of defects in the ovule.
The zygotically active gene GNOM/EMB30 is also thought to be involved in establishing apical-basal polarity18. Mutants divide more symmetrically at the first embryonic division, and can later appear spherical due to lack of patterning. GNOM/EMB30 encodes a protein analogous to a yeast protein that is involved in vesicular transport and necessary for growth (19).
Further downstream both in an epistatic and a chronological sense, are genes governing the development of segments along the apical-basal axis of the embryo. A number of corresponding mutants are known: monopteros, fackel and gurke, corresponding to deletions of the basal, central and apical regions of the embryo. The activity of the corresponding genes divides the embryo into three segments, reliant upon them for their development (20).
The plant body plan also includes a radial pattern of three principal cell layers (Figure 2) arranged in a concentric ring. This patterning is compromised in knolle and keule mutants (20). Knolle mutants have their epidermal (L1) layer disrupted by misoriented cell divisions from the early globular stage (21) (refer to Box 1). Keule mutants have bloated and irregularly arranged epidermal cells recognisable at the globular stage (20). The two mutants differ in seedling morphology (20).
Other mutants change the overall shape of embryos and seedlings, while not affecting the body plan: Such a mutant is fass, although it may have supernumerary cotyledons (20). A change in cell shape effects the aberrant morphology and the short compressed appearance suggests a defect in reorientation of cellulose synthesis, wall softening, vacuole swelling or signalling pathways necessary for growth. Knopf and mickey mutants are locally affected: Their L1 and L3 layers fail to develop properly (20).
As plant embryos express a large number of genes right from the start of their development, and many embryo lethal mutants are known, it's possible that many important patterning genes have not been found yet (22).
Meristem formation and maintenance
The immobility of plant cells and importance of maintaining tissue organisation necessitate that at the meristems certain cells are compelled to proliferate whilst others are stopped from doing so. This pattern is repeated at the next developmental level, the question of which meristematic tissues are allowed to proliferate, discussed in the next section.
Genes have been identified from mutants that influence meristem development: In shoot meristem mutants, no shoot apical meristem is formed (23). In wuschel mutants, meristems are small and terminate prematurely (24). One group of mutants, the clavata mutants, have enlarged meristems.
All meristems above ground level are derived from the shoot apical meristem, initiated early in embryonic development - WUS expression sets in at the 16-cell stage (25), and CLV genes come in before the heart stage to establish the feedback system (26). Many other genes believed to be involved in meristem functions, such as STM, are expressed between the globular and heart stages (27-29). STM activity is required to sustain CLV expression (27).
The genes CLAVATA1 (CLV1), encoding a receptor kinase, CLAVATA2 (CLV2), encoding an accessory protein (30), and CLAVATA3 (CLV3), encoding a signaling peptide (31), have been cloned (32). The genes CLV3 and CLV1 are apparently on the same pathway (33). It appears that SHOOT MERISTEMLESS (STM) (34) and WUSCHEL (WUS) (24) promote meristem proliferation, whilst CLV1 and CLV3 inhibit it. STM antagonizes CLAVATA genes (34). In situ hybridization (26) has shown the anticipated spatial pattern (32,34) of gene expression at meristems (Figure 3): The activity of WUS below the stem cells maintains stem cell identity above, whilst CLV1 and CLV3 limit the size of the meristem where they coincide. WUS product is also required for the expression of CLV3 (26). This is the mechanism of meristem maintenance.
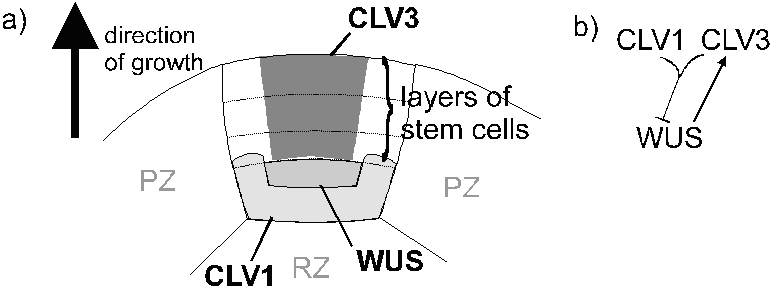 |
| Figure 3: The proposed mechanism of maintenance of meristems. A) Spatial relationship of zones of expression of CLAVATA1 (CLV1), CLAVATA3 (CLV3) and WUSCHEL (WUS). PZ, peripheral zone, RZ, rib zone. Where gene expression is indicated is the central zone. B) Pattern of enhancement and suppression of gene products. |
Apical dominance
Plants have many meristems, for instance one at every leaf node. Most of these are in reserve, covering for potential damage to the apical meristem. They must be suppressed by the growing apex but become active when the dominant apex is removed. The extent of apical dominance is under genetic, physiological and environmental control (e.g. ref. 35).
Polar transport taking auxin from apex to base of the stem is necessary and sufficient for apical dominance (36-39). Cytokinin antagonizes auxin. From such evidence, two models of apical dominance have been suggested - the direct and indirect auxin inhibition models. The direct auxin inhibition model suggests that auxin from the apex is transported to axillary buds where it inhibits growth, but has been rejected because auxin is transported away from axillary meristems and levels increase after relief of apical dominance (40). The indirect model states that the development of vascular tissue directing photoassimilate and nutrients and perhaps cytokinin to the growing apex is favoured as long as most of the auxin comes from there, and hence maintained (41,42).
The auxin transported down from the apical bud and other, dormant, buds probably also has an effect on root patterning. Lateral roots can be initiated by auxin (43,44). Similar developmental principles apply to root as to shoot meristems: A mechanism for maintenance of meristems is required, and similarly, lateral root development must be controlled in a fashion analogous to apical dominance.
Discussion and summary
Any multicellular organism develops by growth and division of its cells. In addition, complex multicellular organisms follow pre-programmed body plans. Animals as one monophyletic group of complex multicellular organisms show determinate development (with few exceptions) according to baupläne. Plants as another such group have adapted to a sessile, terrestrial, photosynthetic life that imposes special requirements on their mechanisms of development, such as the requirements of developmental plasticity.
While the cell wall at first might seem to present particular hurdles to development, it is indeed an important part of it, and we have a working hypothesis of the mechanism of growth at the cellular level: In response to external stimuli, a cell can adjust the polarity of its cell wall to grow in a certain direction. This is believed to be by the direct influence of microtubules on the direction of travel of cellulose synthase rosettes, though other hypotheses are being considered (45). The pH-dependent action of expansin helps to relax the wall when growth takes place.
Plant embryos develop from zygotes by a series of predetermined and closely controlled cell divisions. Some genes contributing to this control have been identified, although their pattern of interaction needs further study. Most of a plant's body plan is laid down from meristems in post-embryonic development, meristems retaining their role as growth controllers throughout the plant's life. In meristems, some cells are allowed to proliferate while others are prevented from doing so. The auxin secreted by the dominant apical bud prevents the development of The stories of maintenance of meristems and apical dominance have a common theme: The development of some units whilst suppressing others.
Indeed it is the cell wall and resulting immobility of cells that makes it possible to have such well-controlled patterns of development. The intricate spatial signalling context of cell differentiation in plants also guards against irregularities in development that animals suffer, such as cancer.
References:
| 1. | Pear, J. R., Kawagoe, Y., Schreckengost, W. E., Delmer, D. P. & Stalker, D. M. 1996. Higher plants contain homologs of the bacterial celA genes encoding the catalytic subunit of cellulose synthase. Proceedings of the National Academy of Sciences USA 93, 12637-42. |
| 2. | Green, P. B. 1962. Mechanism for plant cellular morphogenesis. Science 138, 1404-5. |
| 3. | Ledbetter, M. C. & Porter, K. R. 1963. A "microtubule" in plant cell fine structure. Journal of Cell Biology 19, 239-50. |
| 4. | Baskin, T. I. 2001. On the alignment of cellulose microfibrils by cortical microtubules: a review and a model. Protoplasma 215, 150-71. |
| 5. | Varner, J. E. & Lin, L.-S. 1989. Plant cell wall architecture. Cell 56, 231-9. |
| 6. | Kropf, D. L., Bisgrove, S. R. & Hable, W. E. 1998. Cytoskeletal control of polar growth in plant cells. Current Opinion in Cell Biology 10, 117-22. |
| 7. | Cyr, R. J. 1994. Microtubules in plant morphogenesis: role of the cortical array. Annual Review of Cell Biology 10, 153-80. |
| 8. | Cleland, R. E. 1980. Auxin and H+ excretion: the state of our knowledge. In: Skoog, F. (ed.), Plant growth substances, pp. 71-8. Springer, Berlin. |
| 9. | McQueen-Mason, S. J. & Cosgrove, D. J. 1995 Expansin mode of action on cell-walls - analysis of wall hydrolysis, stress-relaxation and binding. Plant Physiology 107, 87-100. |
| 10. | Fleming, A. J., McQueen-Mason, S., Mandel, T. & Kuhlemeier, C. 1997. Induction of leaf primordia by the cell wall protein expansin. Science 276, 1415-8. |
| 11. | Wiebe, H. H. 1978. The significance of plant vacuoles. BioScience 28, 327-31. |
| 12. | Tucker, E. B. 1982. Translocation in the staminal hairs of Setcreasea purpurea. 1. A study of cell ultrastructure and cell-to-cell passage of molecular probes. Protoplasma 113, 193-201. |
| 13. | Goodwin, P. B. 1983. Molecular size limit for movement in the symplast of the Elodea leaf. Planta 157, 124-30. |
| 14. | Ding, B. 1998. Intercellular protein trafficking through plasmodesmata. Plant Molecular Biology 38, 279-310. |
| 15. | Kropf, D. L., Kloareg, B. & Quatrano, R. S. 1988. Cell-wall is required for fixation of the embryonic axis in Fucus zygotes. Science 239, 187-90. |
| 16. | Torres Ruiz, R. A. & Jürgens, G. 1994. Mutations in the FASS gene uncouple pattern formation and morphogenesis in Arabidopsis development. Development 120, 2967-78. |
| 17. | Ray, S., Golden, T. & Ray, A. 1996. Maternal effects of the short integument mutation on embryo development in Arabidopsis. Developmental Biology 180, 365-9. |
| 18. | Mayer, U., Buettner, G. & Jürgens, G. 1993. Apical-basal pattern formation in the Arabidopsis embryo: Studies on the role of the gnom gene. Development 117, 149-62. |
| 19. | Shevell, D. E., Leu, W. M., Gillmor, C. S., Xia, G., Feldmann, K. A. & Chua, N. H. 1994. EMB30 is essential for normal cell division, cell expansion, and cell adhesion in Arabidopsis and encodes a protein that has similarity to Sec7. Cell 77, 1051-62. |
| 20. | Mayer, U., Torres Ruiz, R. A., Berleth, T., Miséra, S. & Jürgens, G. 1991. Mutations affecting body organization in the Arabidopsis embryo. Nature 353, 402-7. |
| 21. | Lukowitz, W., Mayer, U. & Jürgens, G. 1996. Cytokinesis in the Arabidopsis embryo involves the syntaxin-related KNOLLE gene product. Cell 84, 61-71. |
| 22. | Jürgens, G. 1994. Pattern formation in the embryo. In: Meyerowitz, E. M. & Somerville, C. R. (eds.), Arabidopsis, pp. 297-312. Cold Spring Harbor Press. |
| 23. | Barton, M. K. & Poethig, R. S. 1993. Formation of the shoot apical meristem in Arabidopsis thaliana: An analysis of development in the wild type and in the shoot meristemless mutant. Development 119, 823-31. |
| 24. | Laux, T., Mayer, K. F. X., Berger, J. & Jürgens, G. 1996. The WUSCHEL gene is required for shoot and floral meristem integrity in Arabidopsis. Development 122, 1143-55. |
| 25. | Mayer, K. F. X., Schoof, H., Haecker, A, Lenhard, M., Jürgens, G. & Laux, T. 1998. Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell 95, 805-15. |
| 26. | Schoof, H., Lenhard, M., Haecker, A., Mayer, K. F. X., Jürgens, G. & Laux, T. 2000. The stem cell population of Arabidopsis shoot meristems is maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell 100, 635-44. |
| 27. | Long, J. A. & Barton, M. K. 1998. The development of apical embryonic pattern in Arabidopsis. Development 125, 3027-35. |
| 28. | Aida, M., Ishida, T. & Tasaka, M. 1999. Shoot apical meristem and cotyledon formation during Arabidopsis embryogenesis: interaction among the CUP-SHAPED COTYLEDON and SHOOT MERISTEMLESS genes. Development 126, 1563-70. |
| 29. | Aida, M., Ishida, T., Fukaki, H., Fujisawa, H. & Tasaka, M. 1997. Genes involved in organ separation in Arabidopsis: an analysis of the cup-shaped cotyledon plant. Plant Cell 9, 841-57. |
| 30. | Jeong, S., Trotochaud, A. E. & Clark, S. E. 1999. The Arabidopsis CLAVATA2 gene encodes a receptor-like protein required for the stability of the CLAVATA1 receptor-like kinase. Plant Cell 11, 1925-34. |
| 31. | Fletcher, J. C., Brand, U., Running, M. P., Simon, R. & Meyerowitz, E. M. 1999. Signalling of cell fate decisions by CLAVATA3 in Arabidopsis shoot meristems. Science 283, 1911-4. |
| 32. | Clark, S. E., Williams, R. K. & Meyerowitz, E. M. 1997. The CLAVATA1 gene encodes a putative receptor kinase that controls shoot and floral meristem size in Arabidopsis. Cell 89, 575-85. |
| 33. | Clark, S. E., Running, M. P. & Meyerowitz, E. M. 1995. CLAVATA3 is a specific regulator of shoot and floral meristem development affecting the same processes as CLAVATA1. Development 121, 2057-67. |
| 34. | Clark, S. E., Jacobsen, S. E., Levin, J. Z. & Meyerowitz, E. M. 1996. The CLAVATA and SHOOT MERISTEMLESS loci competitively regulate meristem activity in Arabidopsis. Development 122, 1567-75. |
| 35. | Lejeune, P. & Bernier, G. 1996. Effect of environment on the early steps of ear initiation in maize (Zea mays L). Plant Cell and Environment 19, 217-24. |
| 36. | Went, F. W. 1935. P. Koniklijke Nederl. 38, 752. |
| 37. | Thimann, K. V. 1934. Journal of. Genetics and Physiology 18, 23. |
| 38. | Skoog, F. 1934. Proceedings of the National Academy of Sciences USA 20, 480. |
| 39. | Skoog, F. 1934. Science 79, 256. |
| 40. | Bond, S. & Alderson, P. G. 1993. The influence of apical dominance on the in.vitro multiplication of the rhizome of Alstroemeria. Journal of Horticultural Science 68, 905-10. |
| 41. | Cline, M. G. 1994. The role of hormones in apical dominance - new approaches to an old problem in plant development. Physiologia Plantarum 94, 230-7. |
| 42. | Tamas, I. 1995. Hormonal regulation of apical dominance. In: Davies, P. J. (ed.), Plant hormones, pp. 572-97. Kluwer Academic Publishers. |
| 43. | Malamy, J. E. & Benfey, P. N. 1997. Down and out in Arabidopsis: The formation of lateral roots. Trends in Plant Sciences 2, 390-6. |
| 44. | Reed, R. C., Brady, S. R. & Muday, G. K. 1998. Inhibition of auxin movement from the shoot into roots inhibits lateral root development in Arabidopsis. Plant Physiology 118, 1369-78. |
| 45. | Emons, A. M. C. & Mulder, B. M. 2000. How the deposition of cellulose microfibrils builds cell wall architecture. Trends in Plant Sciences 5, 35-40. |
| 46. | Yeung, E. C. & Meinke, D. W. 1993. Embryogenesis in angiosperms: Development of the suspensor. Plant Cell 5, 1371-81. |
| 47. | Yeung, E. C. & Sussex, I. M. 1979. Embryogeny of Phaseolus coccineus: The suspensor and the growth of the embryo-proper in vitro. Zeitschrift für Pflanzenphysiologie 91, 423-33. |
| 48. | Scheres, B., Wolkenfelt, H., Willemsen, V., Terlouw, M., Lawson, E., Dean, C. & Weisbeel, P. 1994. Embryonic origin of the Arabidopsis primary root and root meristem initials. Development 120, 2475-87. |